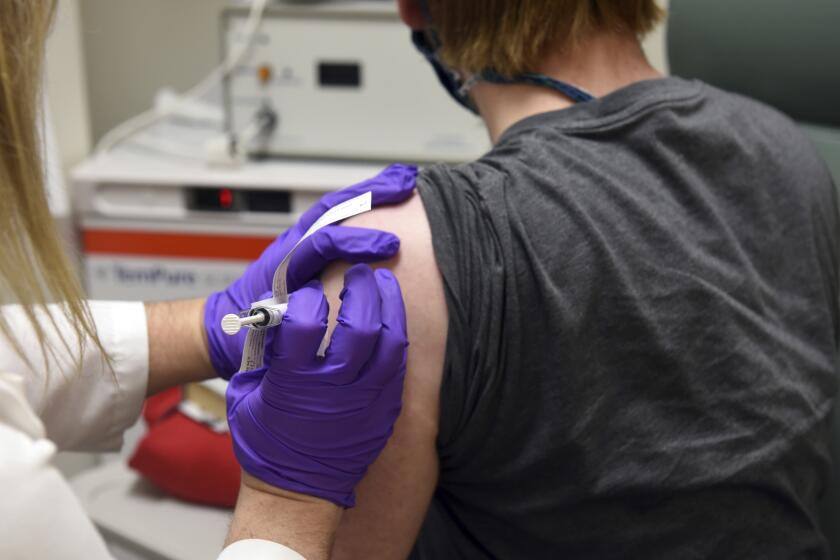
How were COVID-19 vaccines made so fast? Scientists had a huge head start
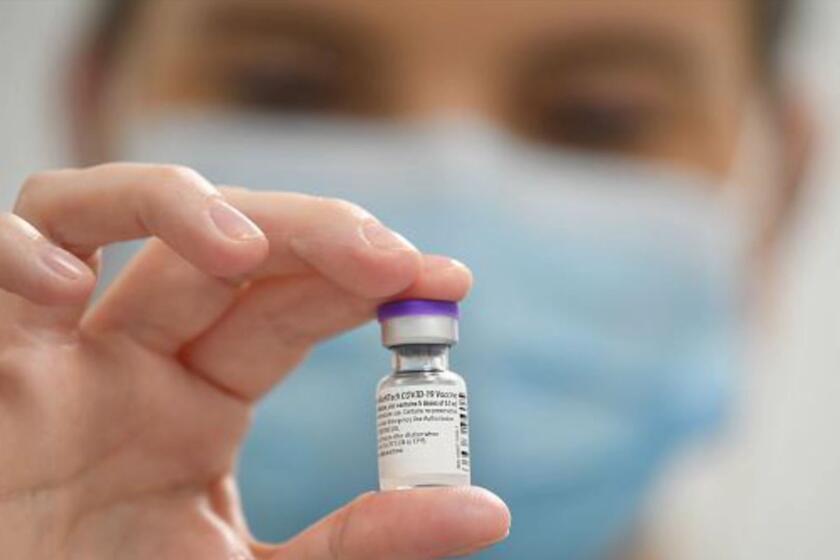
How were scientists able to produce COVID-19 vaccines so quickly without cutting corners? A head start helped â over a decade of behind-the-scenes research that resulted in a new vaccine technology poised for a challenge just as the coronavirus emerged.
âThe speed is a reflection of years of work that went before,â said Dr. Anthony Fauci, the top U.S. infectious disease expert. âThatâs what the public has to understand.â
Creating vaccines and having results from rigorous studies less than a year after discovering a never-before-seen disease is incredible, cutting years off the normal development timeline. But the two U.S. frontrunners are made in a way that suggests speedier development may become the norm â especially if they live up to the promise of their early testing results.
âAbject giddinessâ is how Dr. C. Buddy Creech, a Vanderbilt University vaccine expert, described scientistsâ reactions when separate studies showed the two candidates were about 95% effective.
Federal regulators will consider requests in December by Pfizer and Moderna for emergency use authorization for their coronavirus vaccines.
âI think we enter into a golden age of vaccinology by having these types of new technologies,â Creech said at a briefing of the Infectious Diseases Society of America.
Both shots â one made by Pfizer and BioNTech, the other by Moderna and the National Institutes of Health â are so-called messenger RNA, or mRNA, vaccines, a brand-new technology. U.S. regulators are set to decide this month whether to allow emergency use, paving the way for rationed shots that will start with health workers and nursing home residents.
Billions in company and government funding certainly sped up vaccine development. In addition, the unfortunately huge number of infections meant scientists didnât have to wait long to learn that the shots appeared to be working.
But long before COVID-19 was on the radar, the groundwork was laid in large part by two different streams of research, one at the NIH and the other at the University of Pennsylvania. Scientistsâ investigations into the coronaviruses responsible for the SARS and MERS outbreaks helped too.
âWhen the pandemic started, we were on a strong footing both in terms of the scienceâ and experience handling mRNA, said Dr. Tal Zaks, chief medical officer of Cambridge, Mass.-based Moderna.
When people talk about COVID-19 vaccines, they can sound like theyâre speaking a foreign language. Donât worry! Hereâs your guide to vaccine vocabulary.
Traditionally, making vaccines required growing viruses or pieces of viruses â often in giant vats of cells or, like most flu shots, in chicken eggs â and then purifying them before next steps in brewing shots.
The mRNA approach is radically different. It starts with a snippet of genetic code that carries instructions for making proteins. Pick the right virus protein to target, and the body turns into a mini vaccine factory.
âInstead of growing up a virus in a 50,000-liter drum and inactivating it, we could deliver RNA and our bodies make the protein, which starts the immune response,â said Dr. Drew Weissman, an immunology and vaccine researcher at the University of Pennsylvania.
Fifteen years ago, Weissmanâs lab was trying to harness mRNA to make a variety of drugs and vaccines. But researchers found that simply injecting the genetic code into animals caused harmful inflammation.
Then Weissman and a Penn colleague now at BioNTech, Katalin Kariko, discovered a tiny modification to a building block of lab-grown RNA that let it slip undetected past inflammation-triggering sentinels.
âThey could essentially make a stealth RNA,â said Dr. Philip Dormitzer, Pfizerâs chief scientific officer for viral vaccines.
Other researchers added a fat coating, called lipid nanoparticles, that helped the stealth RNA easily get inside cells and start production of the target protein.
COVID-19 vaccines may not be particularly effective in the elderly residents of long-term care facilities. A look at why they are getting them first.
Meanwhile at the NIH, Dr. Barney Grahamâs team figured out the right target â how to use the aptly named âspikeâ protein on the coronavirusâ exterior to properly prime the immune system.
The right design is critical. It turns out the surface proteins that allow a variety of viruses to latch onto human cells are shape-shifters, rearranging their form before and after theyâve fused into place. Brew a vaccine using the wrong shape and it wonât block infection.
âYou could put the same molecule in one way and the same molecule in another way and get an entirely different response,â Fauci explained.
That discovery was made in 2013, when Graham, the deputy director of NIHâs Vaccine Research Center, and his colleague Jason McLellan were investigating a decades-old failed vaccine against RSV, a childhood respiratory illness.
They homed in on the right structure for an RSV protein and learned genetic tweaks that stabilized the protein in the correct shape for vaccine development. They went on to apply that lesson to other viruses, including researching a vaccine for MERS, a COVID-19 cousin, although it hadnât gotten far when the pandemic began.
âThatâs what put us in a position to do this rapidly,â Graham said in February, before the NIHâs vaccine was first tested in people. âOnce you have that atomic-level detail, you can engineer the protein to be stable.â
Questions of possible insider trading â including at firms developing COVID-19 vaccines â follow large stock sell-offs by executives at Pfizer and Moderna.
Likewise, Germanyâs BioNTech in 2018 had partnered with New York-based Pfizer to develop a more modern mRNA-based flu vaccine. That gave both companies some early knowledge about how to handle the technology.
âThis was all brewing,â Dormitzer said. âThis didnât come out of nowhere.â
In January, shortly after the new coronavirus was reported in China, BioNTech Chief Executive Ugur Sahin switched gears and used the same method to create a COVID-19 vaccine.
Moderna also was using mRNA to develop vaccines against other germs, including the mosquito-borne Zika virus. That research showed promise, but that wasnât moving rapidly since the Zika outbreak had fizzled.
Then, at the NIH, Graham woke up on Jan. 11 to see Chinese scientists had shared the genetic map of the new coronavirus. His team got to work on the right-shaped spike protein. Days later, they sent Moderna that recipe.
And the vaccine race was on.