What we know about AstraZeneca blood clot reports
- Share via
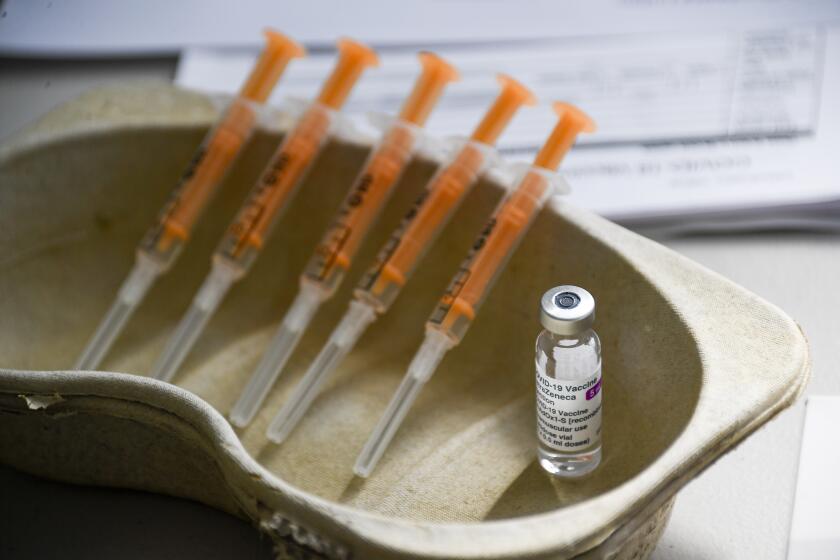
LONDON — German officials have decided to limit the use of AstraZeneca’s coronavirus vaccine in people younger than 60 after more unusual blood clots were reported in a small number of those who had received the shots.
Earlier this month, more than a dozen countries, including Germany, suspended their use of AstraZeneca over the blood clot issue. Most restarted — some with the kinds of restrictions Germany imposed Tuesday — after Europe’s drug regulator said the benefits of the vaccine outweighed the risks of not inoculating people against COVID-19.
The seesawing back and forth on who can take the vaccine has raised concerns that its credibility could be permanently damaged. Here’s a look at what we know — and what we don’t.
What happened in Germany?
Earlier this week, Germany’s medical regulator released new data showing a rise in reported cases of unusual kinds of blood clots in people who recently got a dose of the AstraZeneca vaccine. In response, Health Minister Jens Spahn and state officials agreed only to give the vaccine to people 60 or older unless they were at high risk of developing serious complications from COVID-19.
“It’s about weighing the risk of a side effect that is statistically small but needs to be taken seriously and the risk of falling ill with corona,” Spahn said.
Germany’s medical regulator said its tally of the rare blood clots reported by March 29 had increased to 31. Some 2.7 million doses of AstraZeneca have been administered in Germany so far. Nine of the people died, and all but two of the cases involved women, who were ages 20 to 63, the Paul Ehrlich Institute said.
There also have been some reports of clots from elsewhere, among the tens of millions of people who have received the AstraZeneca vaccine.
What have previous investigations found?
Earlier this month, the European Medicines Agency concluded the AstraZeneca shot did not raise the overall risk of blood clots but could not rule out a link to rare clots and recommended a new warning be added to the vaccine’s leaflet. It did not recommend that any new age restrictions be put in place; the shot is authorized for people 18 and older.
The World Health Organization’s expert committee also evaluated available data for the AstraZeneca vaccine and said the shot was safe and effective. On Wednesday, Dr. Kate O’Brien, who heads WHO’s vaccines department, said they were continuing to review the situation.
It’s normal to continue to look for side effects as new vaccines are rolled out since they are typically tested in tens of thousands of people, but some rare problems may only occur once millions receive the shot.
How can scientists figure out if the vaccine is responsible for the rare blood clots?
“The way to tell if cases are caused by vaccination is to look to see if there is an excess of cases in people who have been vaccinated,” said Dr. Peter English, past chair of the British Medical Assn.’s Public Health Medicine Committee.
That will take some time. It took about a year, for instance, before scientists were able to conclude that a swine flu vaccine was responsible for some cases of narcolepsy in Europe.
The latest twist created another layer of uncertainty for a product already facing dwindling public support in Europe
Adam Finn, a professor of pediatrics at the University of Bristol, said there was no compelling evidence yet that the vaccine was to blame for the rare clots.
“The mechanism by which these blood clotting abnormalities come about, and why they affect this very small proportion of individuals, has still not been properly worked out,” he said in a statement.
The European Medicines Agency is continuing to look closely at two rare types of blood clots reported in people who received at least one dose of the AstraZeneca vaccine. It convened two expert meetings this week to evaluate more data and could update its recommendations for the vaccine in the coming weeks.
In a statement, AstraZeneca said it was analyzing the tens of millions of records for people who had received its vaccine “to understand whether these very rare cases of blood clots ... occur any more commonly than would be expected naturally” among millions of people.
What does this mean for COVID-19 vaccinations?
It’s bad news. Health officials worry the repeated suspensions and restrictions for the AstraZeneca vaccine could undermine confidence in a shot that is key to global efforts to stamp out the pandemic since it’s cheaper and easier to store than some others.
In Norway, which recently extended its suspension of the AstraZeneca vaccine for three weeks, officials say the confusion is prompting a wave of vaccine hesitancy.
Health officials say AstraZeneca’s findings from a U.S. trial of its COVID-19 vaccine may have used ‘outdated information’ and ask for clarification.
The leader of the Norwegian Assn. for General Practice, Marte Kvittum Tangen, told broadcaster NRK that resuming the vaccination with AstraZeneca “will be very difficult if we want the greatest possible vaccination coverage in the population in the long run.”
Finn, of the University of Bristol, said the biggest health threat to the world was currently COVID-19 and that any doubts about the effectiveness of authorized coronavirus vaccines was problematic.
“We need to stay focused,” he said, “on the need to prevent [COVID-19] taking millions more human lives before it is brought under control, and the only effective way to do that is through vaccination.”
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.