With approvals still pending, Britain prepares rollout of COVID-19 vaccines
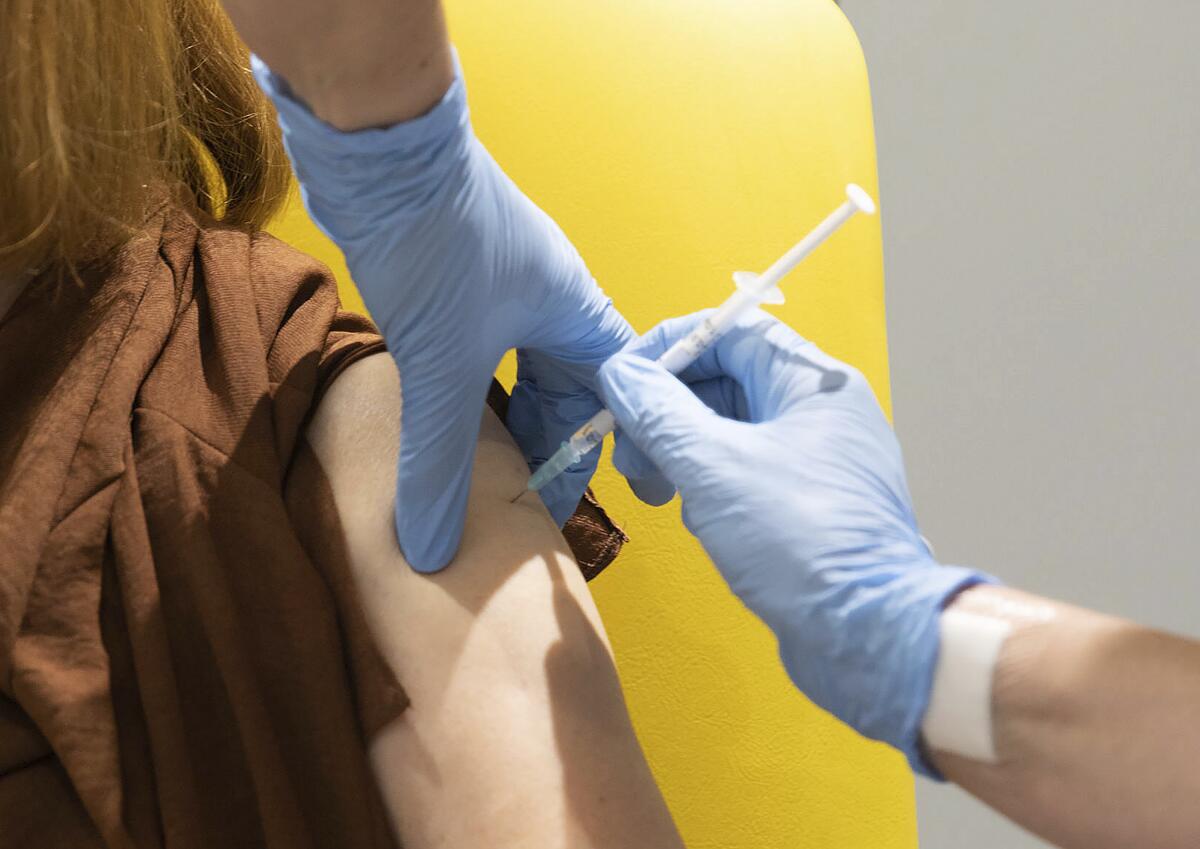
LONDON â Even before regulators have approved a single vaccine, Britain is moving quickly to organize the distribution and delivery systems needed to inoculate millions of its citizens.
With major COVID-19 vaccine candidates showing high levels of protection, including one being co-developed by Oxford University, British officials are cautiously â and they stress âcautiouslyâ â optimistic that life might start returning to normal by early April.
âWith a favorable wind ... we should be able to inoculate, I believe on the evidence Iâm seeing, the vast majority of the people who need the most protection by Easter,â Prime Minister Boris Johnson said Monday after various drugmakers announced encouraging results in recent weeks. âThat will make a very substantial change to where we are at the moment.â
Britain has recorded more than 55,000 deaths linked to COVID-19, the deadliest outbreak in Europe. The pandemic has prevented families from meeting, put 750,000 people out of work and devastated businesses that were forced to shut as authorities tried to control the spread. Englandâs second national lockdown will end Dec. 2, but many restrictions will remain in place.
The British government has agreed to purchase up to 355 million doses of vaccine from seven different producers as it prepares to vaccinate as many of the countryâs 67 million people as possible. Governments around the world are making agreements with multiple developers to ensure that they lock in delivery of the products approved by regulators.
Britainâs National Health Service is making plans to administer 88.5 million vaccine doses throughout England, according to a planning document dated Nov. 13. Scotland, Wales and Northern Ireland are developing their own plans under the U.K.âs system of devolved administration.
U.K. Prime Minister Boris Johnson made financial pledges on schools, housing and infrastructure amid a coronavirus crisis with a death toll of 43,000.
The first to be vaccinated would be healthcare workers and nursing home residents, followed by Britons over 80, according to the document, which was first reported by the London-based Health Service Journal. People under 65 with underlying medical conditions would be next, then healthy people 50 to 65 and finally everyone else 18 and over.
While most of the injections would be delivered at around 1,000 community vaccination centers, about a third would go to 40 to 50 âlarge-scale mass vaccination centers,â including stadiums, conference centers and similar venues, the document indicates.
The NHS confirmed that the document was genuine but said details and target dates were constantly changing because the vaccination program remains a work in progress.
Professor Mark Jit, an expert in vaccine epidemiology at the London School of Hygiene & Tropical Medicine, said Britain had the advantage of a well-developed medical infrastructure that can be used to deliver the vaccine.
With a COVID-19 vaccine drawing closer, public health officials across the country are gearing up for the biggest vaccination effort in U.S. history.
But this effort will be unlike standard vaccination programs that target particular individuals or cohorts.
âThe challenge now is to deliver the biggest vaccine program in living memory in the U.K. and other countries around the world,â Jit said. âWeâre not vaccinating just children or pregnant women like many other vaccination programs. ... Weâre trying to vaccinate the entire U.K. population. And weâre trying to do it very quickly.â
Other European countries are also getting ready, as are the companies that will be crucial to the rollout.
Germanyâs Binder, which makes specialized cooling equipment for laboratories, has ramped up production of refrigerated containers needed to transport some of the vaccines under development. Binder is producing a unit that will reach the ultra-cold temperatures needed to ship the Pfizer vaccine.
The German government has asked regional authorities to get special vaccination centers ready by mid-December. France has reserved 90 million vaccine doses, but has not yet laid out its plan for mass vaccination. A French government spokesman said last week that authorities were working to identify locations for vaccination centers, choose companies to transport vaccines and set the rules for shipping and storage.
In Spain, health workers will get priority, as will residents of nursing homes. Spain hopes to vaccinate some 2.5 million people in the first stage between January and March and have most of the vulnerable population covered by mid-year. The vaccinations will be administered in 13,000 public health centers.
But sticking syringes in peopleâs arms is just the last part of the enormous logistical challenge that the worldwide vaccination campaign will pose.
First, drugmakers must ramp up production, so that there is enough supply to vaccinate billions of people in a matter of months. Then they have to overcome distribution hurdles such as storage of some of the products at minus-94 degrees. Finally, they will need to manage complex supply chains reminiscent of the just-in-time delivery systems that carmakers use to keep their factories humming.
âIt will be the challenge of the century, basically, because of the volumes and everything else which are going to be involved,â said Richard Wilding, a professor of supply chain strategy at Cranfield School of Management. âItâs just the absolute scale.â
Vaccines from three drugmakers are considered leading candidates. Pfizer and Moderna have released preliminary data showing their vaccines were about 95% effective. AstraZeneca on Monday reported interim results of its vaccine developed with Oxford researchers that were also encouraging. Dozens of other vaccines are under development, including projects in China and Russia.
âWe can hear the drumming hooves of the cavalry coming over the brow of the hill, but they are not here yet,â Johnson said.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.











