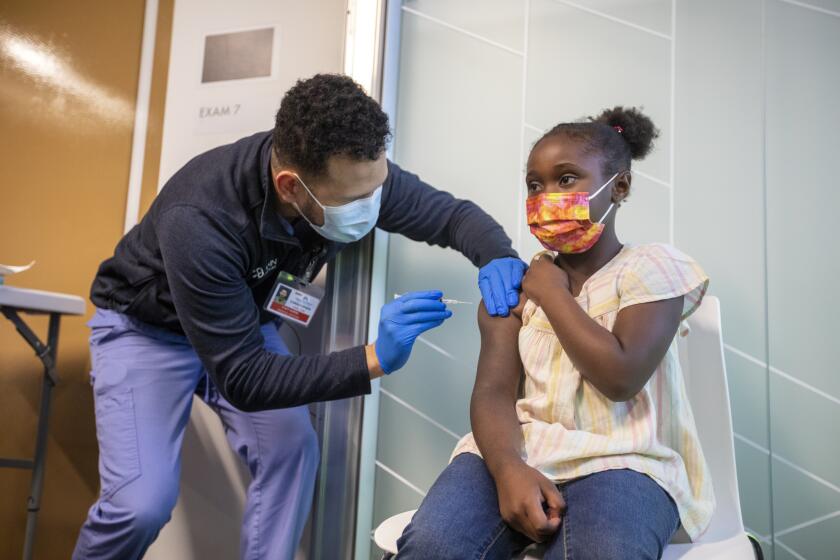
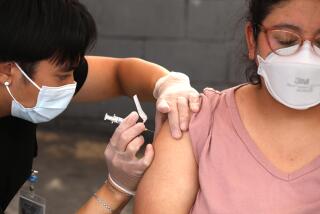
If exposed to the coronavirus, take 3 home tests to boost accuracy, FDA now says

WASHINGTON — If you were exposed to the coronavirus, take three home tests instead of two to make sure you’re not infected, according to new U.S. recommendations released Thursday.
Previously, the Food and Drug Administration had advised taking two rapid antigen tests over two or three days to rule out an infection. But the agency says new studies suggest that protocol can miss too many infections, and could result in people spreading the coronavirus to others, especially if they don’t develop symptoms.
The new guidance applies to people without symptoms who think they may have been exposed. People with symptoms can continue using two tests spaced 48 hours apart.
Thursday’s update reflects the evolving understanding of the accuracy of antigen tests, which are less sensitive than laboratory tests but have become the standard testing approach because of their speed and convenience. Instead of detecting the coronavirus itself, they detect protein traces, known as antigens, similar to rapid flu tests.
The CDC has updated its COVID-19 guidance in an effort to make things less confusing to Americans. Here’s what the agency recommends.
Health officials have repeatedly cautioned that the tests can give false negatives if taken too early. The Centers for Disease Control and Prevention recommends people without symptoms wait to test until five days after an exposure. That’s because it generally takes several days before the antigens reach levels that are detectable via testing with a nose swab.
All 22 home antigen tests on the U.S. market were authorized for emergency use based on preliminary data, while companies and researchers gathered more definitive metrics on their accuracy.
The FDA said its latest decision reflects new information. In a government study, adding a third test improved accuracy from 62% to 79%.