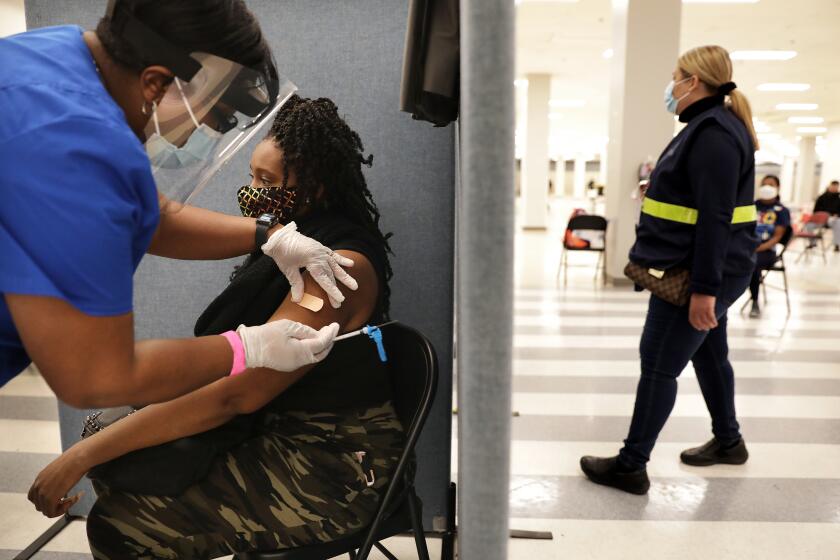
Whatâs different about Johnson & Johnsonâs vaccine that might explain its blood clot risk?
Why do some COVID-19 vaccines seem to pose a small risk of a rare type of blood clot and others donât? Scientists suspect itâs related to the way the vaccines are designed.
All three of the COVID-19 vaccines that have been authorized for use in the U.S. by the Food and Drug Administration â one from Pfizer and BioNTech, one from Moderna and one from Johnson & Johnson â teach the immune system to recognize the coronavirus without exposing the body to the real thing.
The vaccines pull this off by showing the immune system the spike proteins that stud the coronavirus surface.
A spike protein on its own isnât dangerous. But since itâs foreign, the immune system will develop antibodies against it. Then, if a vaccinated person encounters an actual coronavirus, those antibodies will be ready to neutralize the spike proteins â along with the rest of the virus particle theyâre attached to.
Instead of injecting a person with a real-life spike protein, the vaccines deliver a recipe for making spike proteins inside the body. Human cells follow that recipe after immunization â a process that requires one dose of the Johnson & Johnson vaccine and two doses of the Pfizer and Moderna offerings.
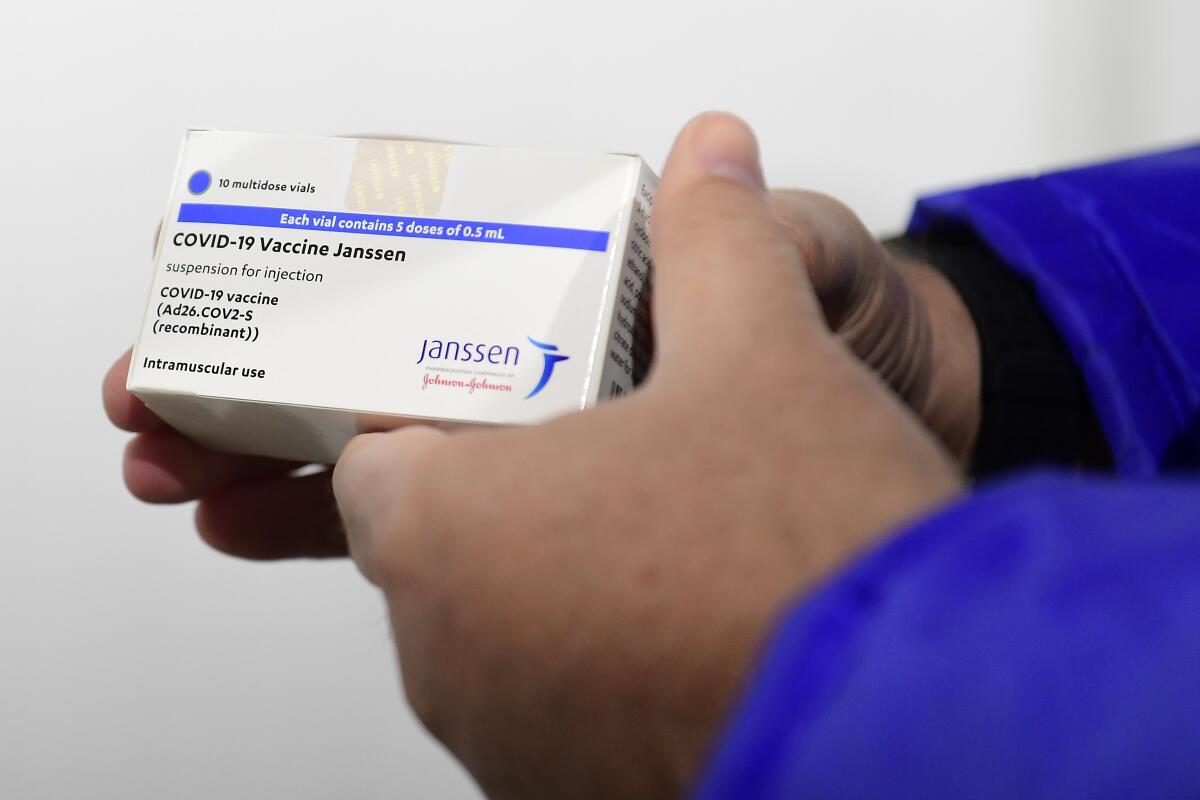
What really differentiates the J&J vaccine from the others is the vehicle it uses to deliver its genetic cargo. That vehicle is an adenovirus â a type of virus that can cause cold-like symptoms. The one J&J uses has been genetically modified so that it canât copy itself and make people sick.
AstraZenecaâs two-dose COVID-19 vaccine â which is not authorized for use in the U.S. â also employs an adenovirus. And it also has been linked to a rare condition that causes blood clots.
Two COVID-19 vaccines have now been linked to a risk of developing blood clots. Scientists donât know whether the vaccines are to blame.
To some experts, that looks like more than a coincidence.
âItâs plainly obvious to us already that what weâre seeing with [the Johnson & Johnson] vaccines is very similar to what weâre seeing with the AstraZeneca vaccine,â said Dr. Peter Marks, the FDAâs chief of drug evaluation.
As scientists dig into the possible ways an adenovirus could trigger a biological process that ends in a life-threatening blood clot, they will study the patients who have wound up with a clot after receiving either vaccine, Marks added.
So far, that unlucky group includes seven of the roughly 7.5 million people in the U.S. who got the Johnson & Johnson vaccine and several dozen people in Europe who received at least one dose of the AstraZeneca vaccine.
Officials at the European Medicines Agency, the drug regulator there, strongly suspect the AstraZeneca vaccine is responsible for the blood clots. The EMA said, however, the benefits of the vaccine still outweighed the risks. (That assessment also applies to the Johnson & Johnson vaccine, which the agency authorized in March but hasnât yet put into widespread use.)
Reports of blood clots among those who got Johnson & Johnsonâs COVID-19 vaccine may make some recipients anxious. Hereâs how to handle those feelings.
Itâs too early for anyone to say with certainty that the blood clots experienced by those who received the Johnson & Johnson vaccine were caused by the shots themselves. U.S. officials have recommended a pause in use of the vaccine while investigators dig into the problem.
Scientists do have some clues about how a vaccine that uses an adenovirus could wind up triggering a rare blood clotting disorder.
Some of them believe the seven women may have suffered a condition called autoimmune thrombocytopenia. Usually, it happens because the immune system mistakenly attacks a patientâs blood platelets, the cells the body uses to form clots. Paradoxically, patients with the condition also develop dangerous blood clots in the brain, lungs, legs or abdomen.
A study of 11 patients in Europe who developed blood clots after getting the AstraZeneca vaccine found that all of them had high levels of antiplatelet factor 4 antibodies â a sign that they, too, might have had autoimmune thrombocytopenia.
Safety monitors have not turned up cases of these rare blood clots in people with low platelet counts after receiving the Pfizer-BioNTech or Moderna vaccines, which use mRNA technology instead of adenoviruses. The instructions for making the spike protein are encoded in messenger RNA and protected by a tiny ball of fat.
Times staff writer Melissa Healy contributed to this report.