Why the long-awaited Ebola vaccine wonât end the Congo outbreak

When Ebola broke out in the Democratic Republic of the Congo a year ago, the global stockpile of a long-anticipated vaccine was 300,000 doses.
At the time, that seemed like plenty.
But as the virus spreads from the epicenter and threatens to explode across the region, the supply of Merckâs newly developed vaccine â once expected to function as a silver bullet â is dwindling, and likely to burn out before the outbreak does.
Officials have gone head-to-head in a bitter clash over the next line of attack. This week the countryâs health minister stepped down rather than bow to international pressure to also start using another vaccine that is much more experimental.
He had banned its use over doubts about its effectiveness. The president, Felix Tshisekedi, was widely expected to lift the ban by the end of this week. The manufacturer, Johnson & Johnson, says it has 1.5 million doses on hand and is ready to start sending them to the region.
Even if there were enough lifesaving vaccines to go around, the regionâs violent conflict has made it virtually impossible for health workers to deliver the shots to every relative and neighbor of each Ebola victim.
âThereâs a level of panic lurking just below the surface â or maybe itâs above the surface now,â said J. Stephen Morrison, senior vice president of the Center for Strategic and International Studies in Washington.
âNo one imagined the level of violence in Ituri and North Kivuâ â two eastern provinces â âwould be so extensive that they wouldnât be able to contain this. Now, vaccines have become that much more important in containing the outbreak,â he said.
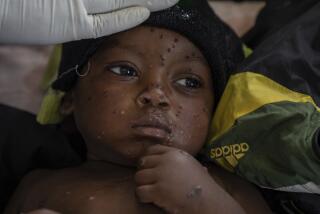
The Ebola virus causes leaky blood vessels and circulatory failure, which starves the bodyâs organs of oxygen â leading to shock and multiple organ failure. It spreads directly from person to person through infected blood, sweat and vomit.
For people who are already infected with the virus, a vaccine canât help.
During the last Ebola outbreak, in West Africa in 2014 and 2015 , there was no vaccine immediately available, a reality that left every person susceptible. By the end, 28,600 people were infected and more than 11,000 died.
But that epidemic eventually became a testing ground for the Merck vaccine, which had been under development for more than a decade and was deployed to Guinea, where it showed positive results.
The vaccine â called V920 â remains unlicensed but appears to be 97.5% effective, according to early data from the current outbreak.
âThese vaccines were the silver lining of the 2014-2015 epidemic, and now theyâre tangled up in the internal chaos. Itâs a bit of a mess,â Morrison said.
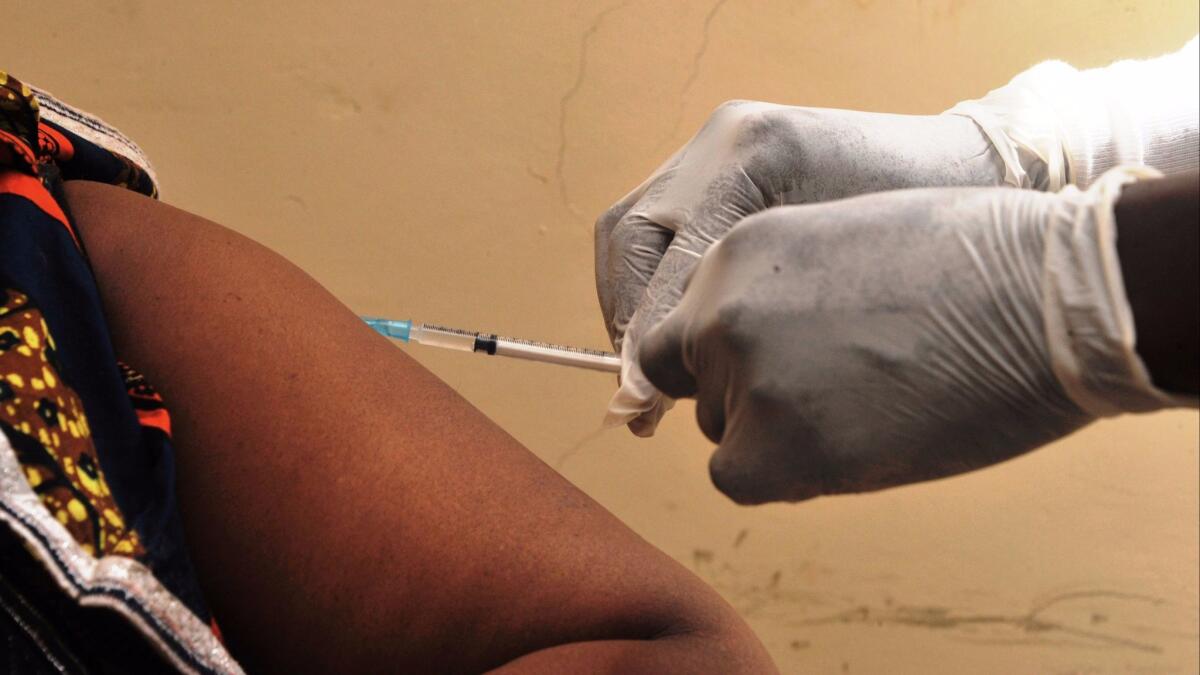
Health workers have administered more than 150,000 doses of the vaccine using a technique called ring vaccination, which prioritizes people who have had exposure to an Ebola victim, followed by expanding circles of their other contacts.
Ideally, the technique barricades the virus and snips each strand of the outbreakâs expanding web. But in a region marked by mass migration, less than a third of all confirmed cases have been linked back to known contacts, according to Doctors Without Borders.
Merck, which has 245,000 doses currently available for shipment from temperature-controlled U.S. warehouses, hopes to triple the cumulative supply to about 900,000 doses over the next 18 months. But as the outbreak spreads, some models predict the current stockpile could be depleted by the fall.
Production of the vaccine takes about one year â which means the region could see a several-month gap before a new vaccine lot is ready.
âWe do project that we are going to run out of vaccine,â Dr. Robert Redfield, the director of the U.S. Centers for Disease Control and Prevention, told lawmakers in testimony last month.
A World Health Organization emergency committee directly acknowledged the shortage of the Merck vaccine supply in a sharply worded statement, calling the current stockpile âinsufficientâ and asking countries and manufacturers to âimmediately take all measures to increase supplies.â
Merck says it is moving production of the vaccine from the U.S. to a new plant in Germany that has not yet gained regulatory approval to ramp up manfuacturing.
For now, production will be split between the two small-scale locations, limiting output.
In response to the shortage, WHOâs Strategic Advisory Group of Experts on Immunization, or SAGE, recommended in May that health officials divide each of the remaining doses by at least half in order to immunize more people.
Merck officials are displeased with the dose-splitting approach, according to two health experts with ties to the company.
Pamela Eisele, a spokeswoman for Merck, said the data showing the vaccine is effective are based on a dose of 1 milliliter. âHowever, we fully recognize the public health need and the exceptional circumstances that led to the interim recommendation SAGE has made, and we respect that decision,â she said.
In May, WHO also recommended use of the Johnson & Johnson vaccine.
Scientists at the London School of Hygiene and Tropical Medicine designed a protocol for getting them to those in need, according to Dr. Peter Piot, the director of the school.
But health officials in the Democratic Republic of Congo resisted using the vaccine.
Their biggest concern is whether it works. Tests in about 6,000 people have shown that it is safe and that it stimulates production of antibodies against the virus. But there is too little data to know its efficacy.
Oly Ilunga Kalenga, the health minister who banned it, was also concerned that the vaccine is administered in two doses, more than six weeks apart. Keeping track of everybody who has been vaccinated â let alone persuading them to return for second shots â is all but impossible in war-torn Congo.
Kalenga also sided with health workers who worry that introducing a second vaccine could undermine public awareness campaigns that are already contending with deep-rooted distrust of authority.
More than a quarter of residents donât believe the outbreak is real, and many people see vaccines as a plot to spread a virus, not eradicate it.
International health experts acknowledge that using unproven vaccines is less than ideal but say there may be no other choice.
âItâs a question of weighing concerns about acceptance and trust against the need for preventing disease,â said Josh Michaud, the associate director of global health policy at Kaiser Family Foundation in Washington. âItâs a balancing act, and as the outbreak expands, the calculation that goes into that equation may change.â
Last week, it did. The outbreak reached Goma, less than a mile from the Rwandan border, prompting officials to declare the epidemic an emergency of international concern. From the bustling city of 2 million people, flights, ferries and buses fan out across central Africa.
Almost immediately, Tshisekedi, the president, consolidated executive control over the outbreak response.
In his resignation letter, the health minister wrote that it was âfanciful to think that the new vaccine proposed by actors who have shown an obvious lack of ethicsâ could have a meaningful impact on containing the outbreak.
But scientists welcomed the opportunity to test the Johnson & Johnson vaccine.
âThe only way to get the key data is to test it in large numbers of the population, and see how it stacks up against the virus,â said Carleigh Krubiner, a policy fellow at the Center for Global Development in Washington.
âWe could have another vital tool in our toolbox for future outbreaks â we just donât know yet.â